Human iPSC-Derived Sensory Neuron Progenitors
iPSC-derived sensory neuron progenitors
We’ve used our expertise in neural differentiation to bring you human dorsal root ganglion (DRG) neurons derived from induced pluripotent stem cells (iPSCs). Axol Human iPSC-Sensory Neuron Progenitors are derived from integration-free iPSCs and have been differentiated to neurons using small molecules. We also offer a fully optimized cell culture system including tailored Sensory Neuron Maintenance Medium and coating reagents to promote the viability and maturation of sensory neurons for endpoint assays on glass or plastic.
Our iPSC-derived sensory neurons express several voltage-gated sodium ion channels and transient receptor potential (TRP) ion channels that play a key role in nociception. These include sodium ion channels Nav1.7 and the DRG-specific, TTX-resistant channels, Nav1.8 and Nav1.9 as well as the temperature-sensitive, TRPV1 and TRPM8, and TRPA1, a sensor of pungency, bitterness and cold.
Axol iPSC-Derived Sensory Neuron Progenitors are available in large batch sizes for reliable and consistent results in high-throughput screening assays. The cells are also suitable for investigating disorders of the peripheral nervous system and chronic pain as well as drug targets for pain relief.
Make Mature Functional Sensory Neurons Ready for Your Assay in just Three Weeks Get the Maturation Maximizer Supplement Now!
Maximizer MEA functional dataiPSC-derived sensory neuron progenitors
| Cat. No. | Product Name | Starting Material | Quantity |
|---|---|---|---|
| ax0055 | Human iPSC-Derived Sensory Neuron Progenitors (Male) | Cord Blood CD34+ Cells | 500,000 cells |
Sensory neuron culture media and reagents
| Cat. No. | Product Name | Product Description | Quantity |
|---|---|---|---|
| ax0060 | Sensory Neuron Maintenance Medium | Basal medium for iPSC-derived Sensory Neurons and Progenitors | 250 ml |
| ax0058 | Sensory Neuron Maturation Maximizer Supplement (New) |
Medium supplement for accelerating maturation of sensory neurons (100X concentrate) | 4 x 250uL vials of Maturation Maximizer Supplement |
|
|
|
|
|
| ax139800 |
BDNF |
Recombinant Human Brain-Derived Neurotrophic Factor |
10 μg / 100 μg |
| ax139855 |
GDNF | Recombinant Human Glial-Derived Neurotrophic Factor |
10 μg / 100 μg |
| ax139789 |
NGF |
Recombinant Human Nerve Growth Factor |
20 μg / 100 μg / 500 μg / 1 mg |
| ax139811 |
NT-3 | Recombinant Human Neurotrophin-3 |
10 μg / 100 μg / 500 μg / 1 mg |
|
|
|
|
|
|
ax0053 |
SureBond-XF |
Xeno-free coating reagent (200X concentrate) |
1 mL |
| Please note: Axol recommends Poly-D-lysine, available from Sigma-Aldrich Cat. No. P7405, as an additional coating substrate for long term culture of the sensory neurons. |
Cell and Medium Kits
| Cat. No. | Product Name | Product Description |
|
|---|---|---|---|
| ax0157 | Human iPSC-derived Sensory Neuron Maximizer Kit | All in one starter kit including 1 vial of the sensory neuron progenitors, 1 bottle of Sensory Neuron Maintenance Medium, the Maturation Maximizer supplement, required growth factors, SureBond-XF coating substrate |
|
| ax0158 | Sensory Neuron Maturation Maximizer Medium Kit |
Sensory Neuron Maintenance Medium with the Maturation Maximizer Supplement |
RNA Sequencing Data
Axol has performed in-depth RNA-seq analysis and characterization of the mature Sensory neurons of a Nociceptor phenotype which are generated using the Axol Sensory neuron kit.
Sensory neurons are highly identified as having key roles in both Pain and Itch sensation and as such have been well characterized for their expressed gene sets.
Our RNA-seq data clearly shows the nociceptor neurons achieved and express typical canonical markers of sensory neurons such as TAC1 (substance P/neurokinin A precursor), RUNX1, SCN9A (NaV1.7), SCN10A (NaV1.8) and P2X3. As well as others.
The accompanying data shown characterizes the transcriptome of IPSC-sensory neurons during their differentiation from IPSC-Sensory progenitor to IPSC-sensory neuron and shows grouping and prevalence of key critical genes.
We note that statistically significant levels of SCN10A (NaV1.8) were not detected by the microarray, but it has been shown that a low level of expression can be observed by a more sensitive qPCR-based expression assay and electrophysiology methods.
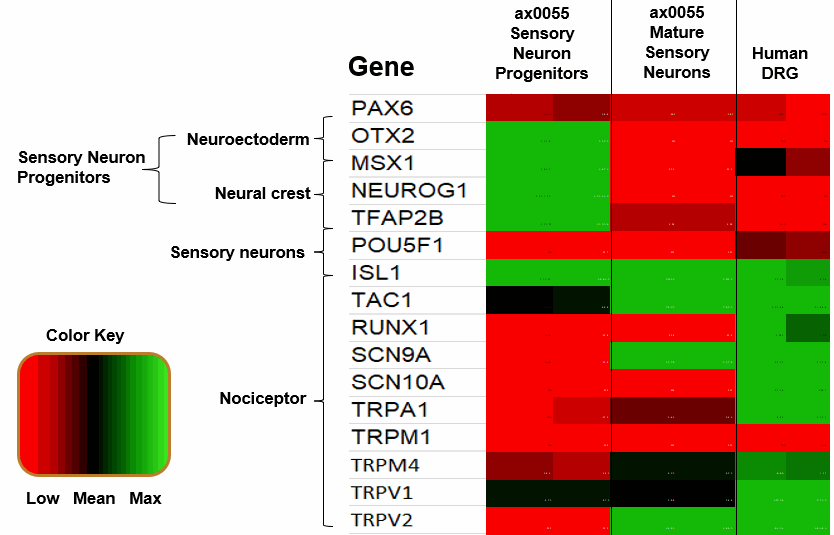
Relative expression levels of key marker genes from Progenitor to Nociceptor, the heatmap displaying gene correlations observed in cells across the populations.
The median expression levels of each gene across replicates are mean centred and scaled to unit variance.
Phase contrast
Phase contrast images show the differentiation and maturation of Axol Human iPSC-derived Sensory Neuron Progenitors over two weeks after thawing and treatment with mitomycin C.

Phase contrast images of Axol Human iPSC-derived Sensory Neuron Progenitors The cells were plated on SureBond-XF in Neural Plating-XF Medium. The cells were then treated with mitomycin C two days after thawing and cultured in the supplemented Sensory Neuron Maintenance Medium. (iPSC-sensory neurons should be culture for a minimum of 3 weeks prior to performing endpoint assays.)
Sodium ion channel expression
Sodium channel RNA expression analysis by cDNA PCR
Axol iPSC-derived Sensory Neuron Progenitors show RNA expression of all three voltage-gated sodium ion channels, Nav1.7, Nav1.8 and Nav1.9.
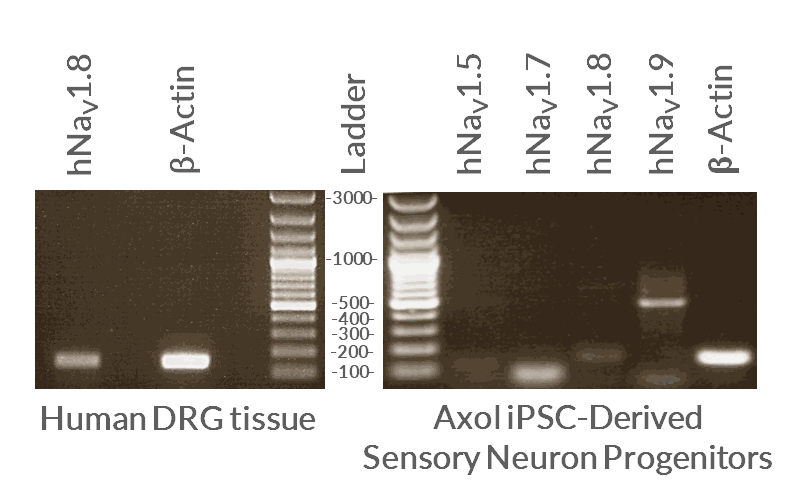
cDNA from iPSC-derived Sensory Neurons was compared to cDNA from human tissue from the dorsal root ganglion (DRG). PCR analysis (40 cycles; 55oC ) confirmed the mRNA expression of SCN9A (82 bp, hNav1.7), SCN10A (149 bp, hNav1.8) and SCN11A (464 bp, hNav1.9) in Axol iPSC-derived sensory neurons. SCN5a (237 bp, hNav1.5) was included as a negative control. Data provided by Dr Edward Emery (University College London).
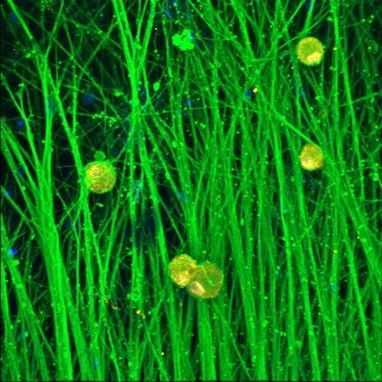
Nav1.7 immunocytochemistry
Axol Human iPSC-derived Sensory Neurons express Nav1.7, a key sodium ion channel in DRG sensory neurons that plays an important role in peripheral pain sensation.
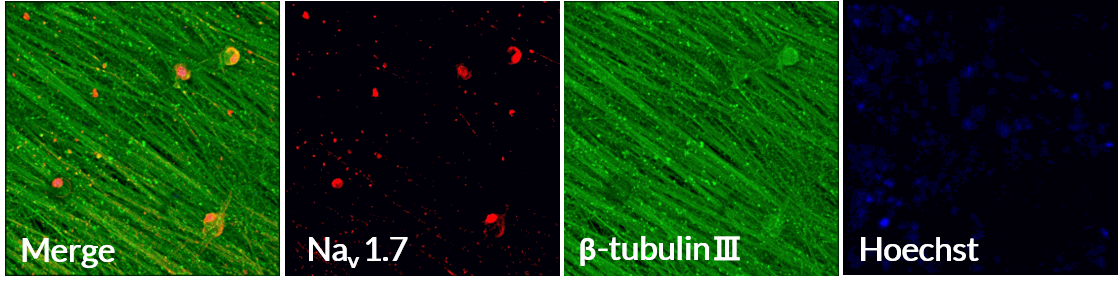
Axol Human iPSC-derived Sensory Neurons express the nociceptive voltage-gated sodium ion channel Nav1.7 on a multi-electrode array (MEA) (Alpha MED Scientific). Nav1.7 (red), β-tubulin Ⅲ (green), Hoechst (blue). Data provided by Prof. Ikuro Suzuki (Tohoku Institute of Technology).
Nav1.7 and Nav1.8 immunocytochemistry
Mature iPSC-derived sensory neurons express the sodium ion channels, Nav1.7 and Nav1.8, and neuronal marker, doublecortin (DCX).
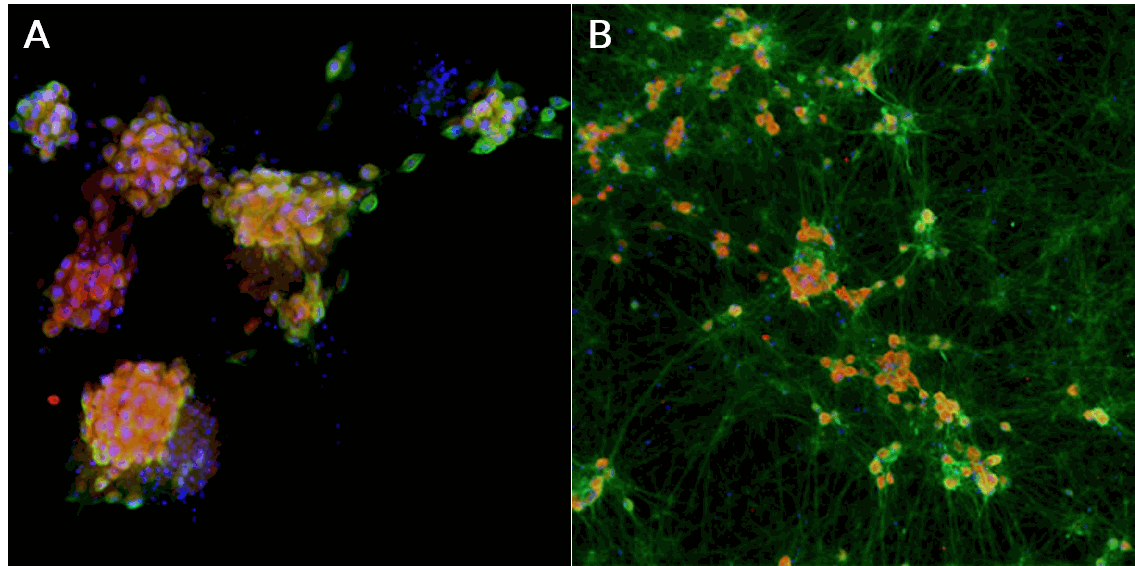
Axol Human iPSC-derived Sensory Neurons show positive staining for the nociceptive voltage-gated sodium ion channels A) Nav1.7 (green), Nav1.8 (red), DAPI (blue), and B) Nav1.8 (red) and doublecortin (green).
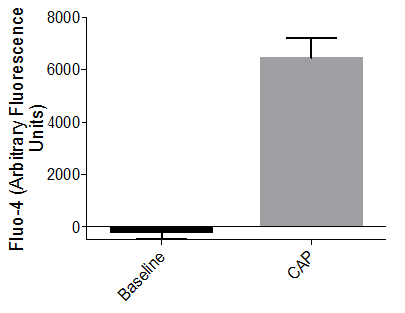
Sodium channel–mediated response to potassium chloride
Axol iPSC-derived Sensory Neurons have been shown to elicit a calcium response to the treatment of KCl at the soma signifying the presence of sodium channels.
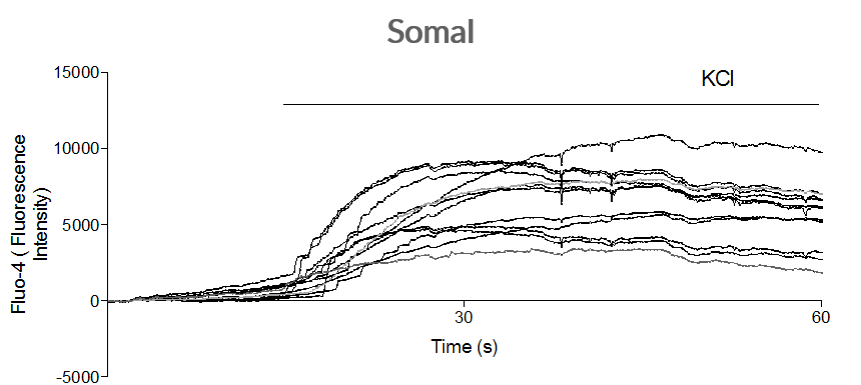
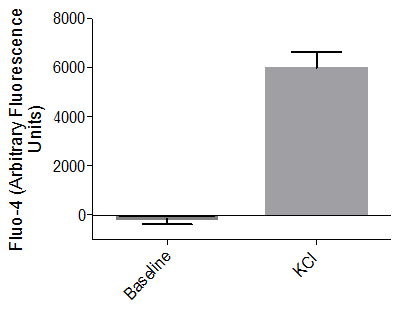
TTX-resistant Nav1.8 and Nav1.9
Nociceptive sensory neurons are unique in that they contain voltage-gated inward current sodium channels (Nav 1.8 and Nav 1.9) that are resistant to tetrodotoxin (TTX). The presence of these TTX-resistant ion channels was confirmed in Axol iPSC-derived sensory neurons.
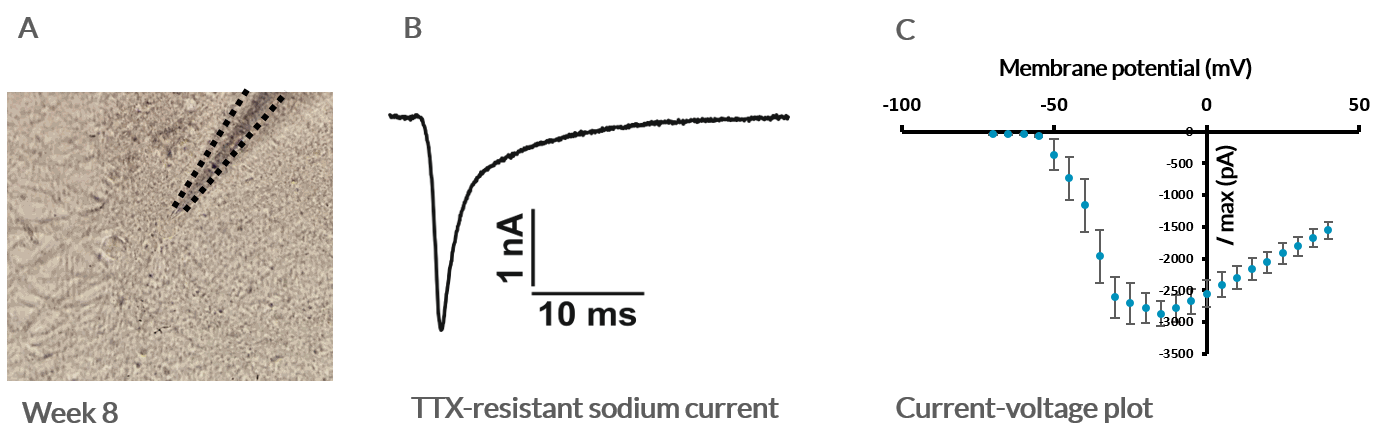
Electrophysiological characterization of Axol Human iPSC-derived Sensory Neurons using patch clamp. A) Phase contrast image of iPSC-derived sensory neurons; B) Example of a sodium-current elicited by a voltage step from -100 mV to -25 mV in the presence of tetrodotoxin (0.5 mM); C) Current-voltage plot of averaged Na-currents recorded from iPSC-derived sensory neurons in the presence of TTX (n=9). Data provided by Dr Edward Emery (University College London).
Transient receptor potential ion channel expression
TRPA1 immunocytochemistry
Axol Human iPSC-derived Sensory Neurons express TRPA1, the ion channel involved in sensing cold, pungency and bitterness.
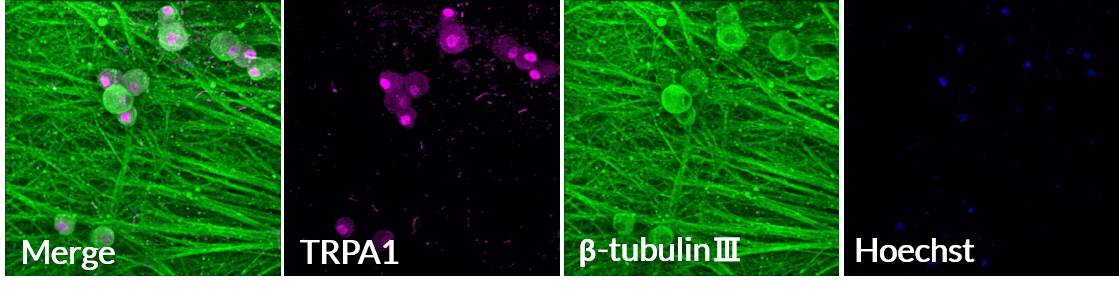
Axol Human iPSC-derived Sensory Neurons express the TRPA1 ion channe on a multi-electrode array (MEA) (Alpha MED Scientific). TRPA1 (purple), β-tubulin Ⅲ (green), Hoechst (blue). Data provided by Prof. Ikuro Suzuki (Tohoku Institute of Technology).
TRPA1–mediated response to allyl isiothiocyanate
Axol iPSC-derived sensory neurons show a short burst of firing after the application of allyl isiothiocyanate (AIT), which indicates the presence of TRPA1 channels.
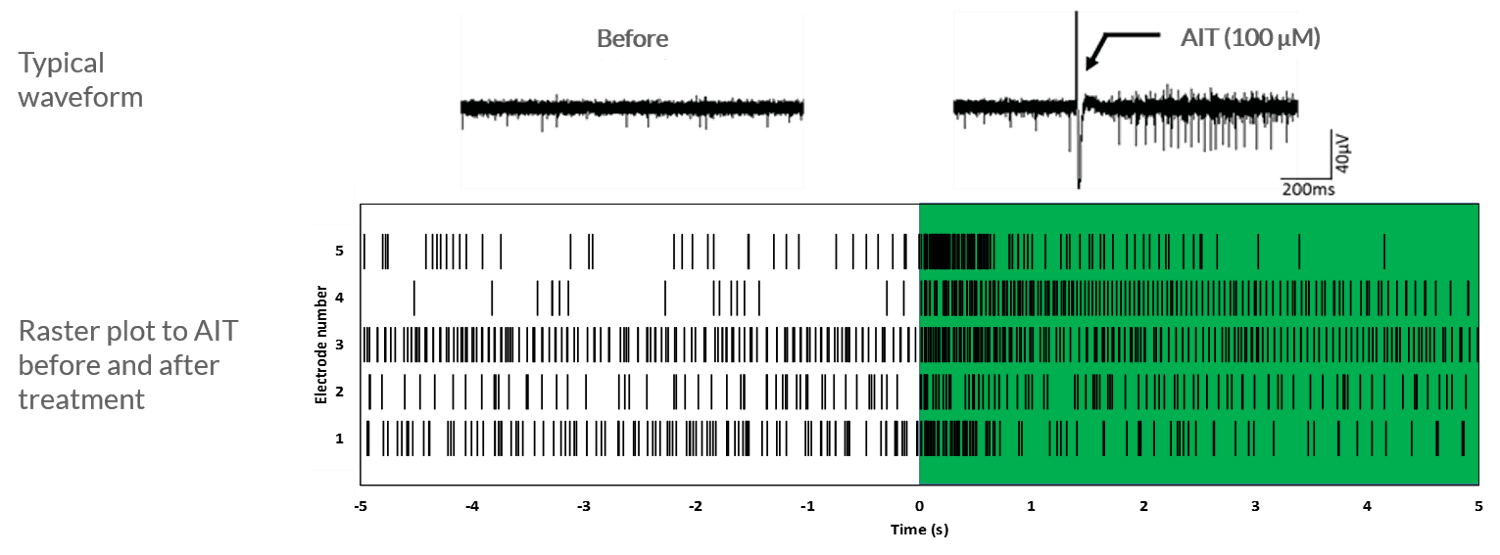
Axol human iPSC-derived sensory neurons show a short burst of firing after the application of 100 μM allyl isothiocyanate. The cells were cultured at 5.0 × 105 cells/cm2 on 64-channel MED-P515A MEA chips (Alpha MED Scientific). This rastor plot shows the firing frequency of sensory neurons before and after the application of 100 μM allyl isothiocyanate (AIT). A short burst of firing after the application of AIT is observed, as expected. Data provided by Prof. Ikuro Suzuki (Tohoku Institute of Technology).
TRPV1 immunocytochemistry
Axol Human iPSC-derived Sensory Neurons express TRPV1, the ion channel involved in sensing heat.
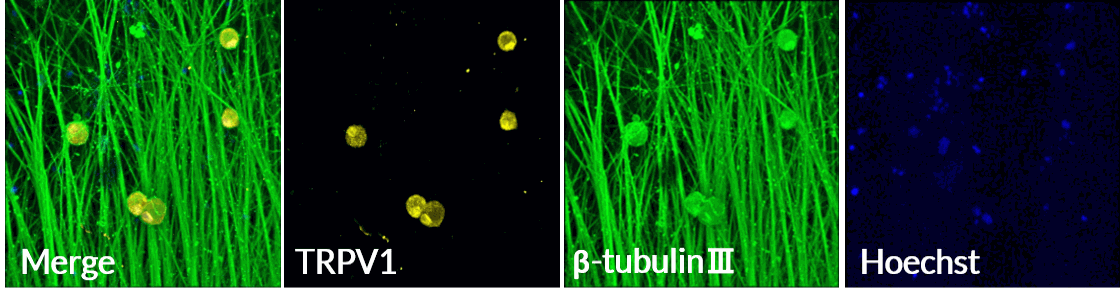
Axol Human iPSC-derived Sensory Neurons express the TRPV1 ion channel on a multi-electrode array (MEA) (Alpha MED Scientific). TRPV1 (yellow), β-tubulin Ⅲ (green), Hoechst (blue). Data provided by Prof. Ikuro Suzuki (Tohoku Institute of Technology).
TRPV1–mediated response to capsaicin
Axol iPSC-derived Sensory Neurons have been shown to elicit a calcium response to the treatment of capsaicin at both the soma and the axon. This indicates that the iPSC-derived Sensory Neurons express TRPV1 and can sense heat.
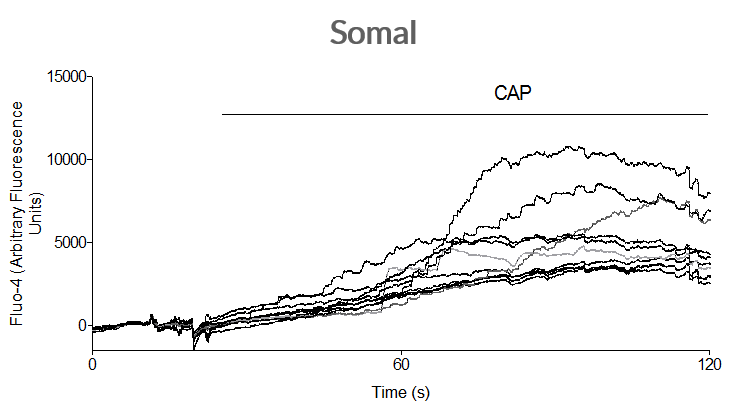

Axol iPSC-derived sensory neurons have been shown to increase neuronal firing after the application of capsaicin, which indicates the presence of TRPV1 channels.
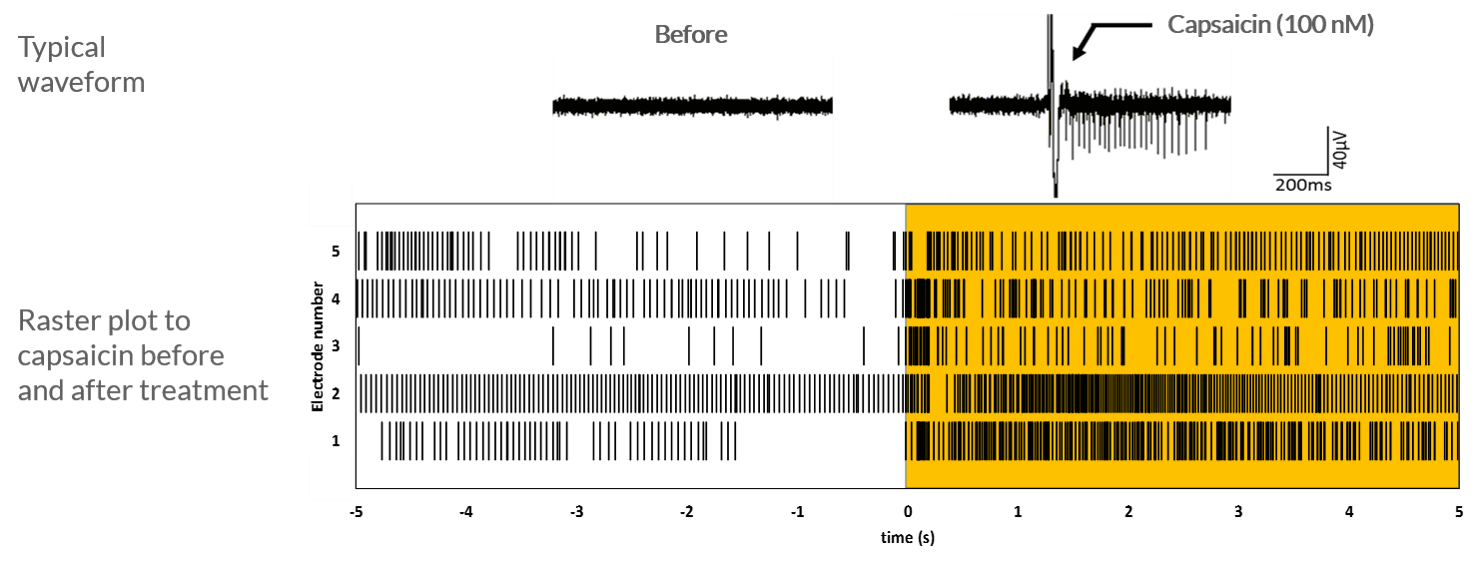
Axol human iPSC-derived sensory neurons show sustained firing after the application of 100 nM capsaicin. The cells were cultured at 5.0 × 105 cells/cm2 on 64-channel MED-P515A MEA chips (Alpha MED Scientific). This rastor plot shows the firing frequency of sensory neurons before and after the application of 100 nM capsaicin. Sustained firing is observed after the application of capsaicin. Data provided by Prof. Ikuro Suzuki (Tohoku Institute of Technology).
TRPV1-mediated response to temperature
Axol iPSC-derived Sensory Neurons subjected to an increase in temperature at day 14 and 21 results in an increase in neuronal firing. This suggests the expression of TRPV1.
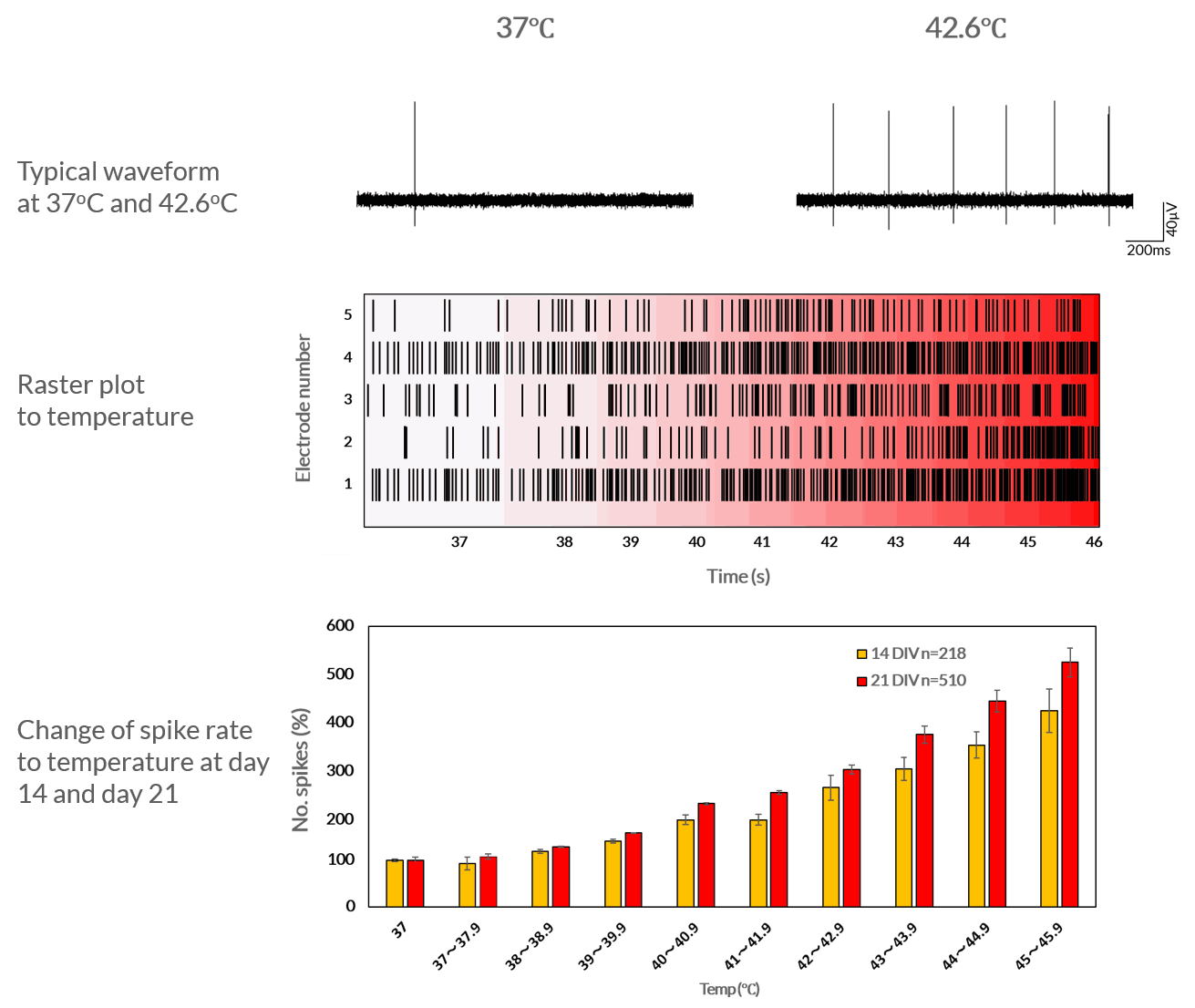
Axol human iPSC-derived sensory neurons show an increase in neuronal firing in response to temperature The cells were cultured at 5.0 × 105 cells/cm2 in a 37°C , 5% CO2 atmosphere on 64-channel MED-P515A MEA chips (Alpha MED Scientific). Spontaneous and evoked extracellular field potentials were acquired at a sampling rate of 20 kHz/channel and signals were high-pass filtered at 100 Hz. Firing analyses and spike sortings were performed using Mobius software (Alpha MED Scientific) on days 14 (n=218) and 21 (n=510) (Alpha MED Scientific). Data provided by Prof Ikuro Suzuki (Tohoku Institute of Technology).
TRPM8-mediated response to menthol
Axol iPSC-derived sensory neurons show an increase in firing frequency after the application of menthol, which indicates the presence of TRPM8 channels.
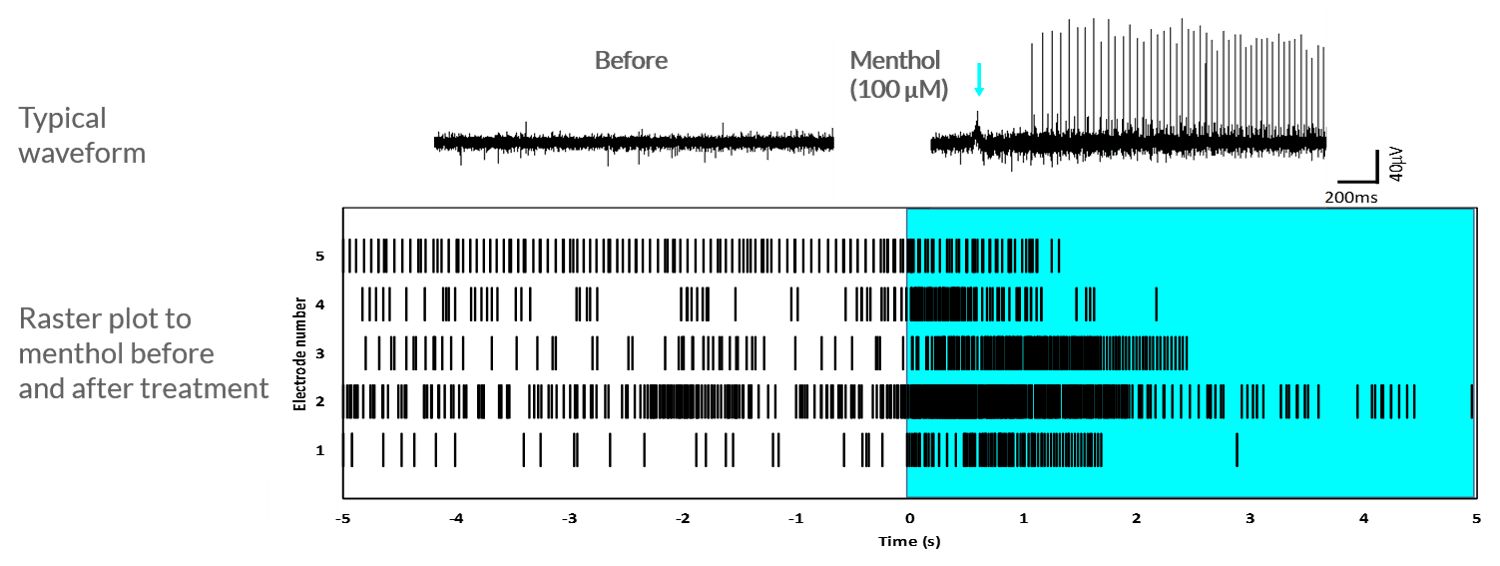
Axol human iPSC-derived sensory neurons show an increase in firing frequency after the application of 100 μM menthol. The cells were cultured at 5.0 × 105 cells/cm2 on 64-channel MED-P515A MEA chips (Alpha MED Scientific). This rastor plot shows the firing frequency of sensory neurons before and after the application of 100 μM menthol. Data provided by Prof. Ikuro Suzuki (Tohoku Institute of Technology).
Electrical activity
Axol Human iPSC-derived Sensory Neuron Progenitors display electrical activity.
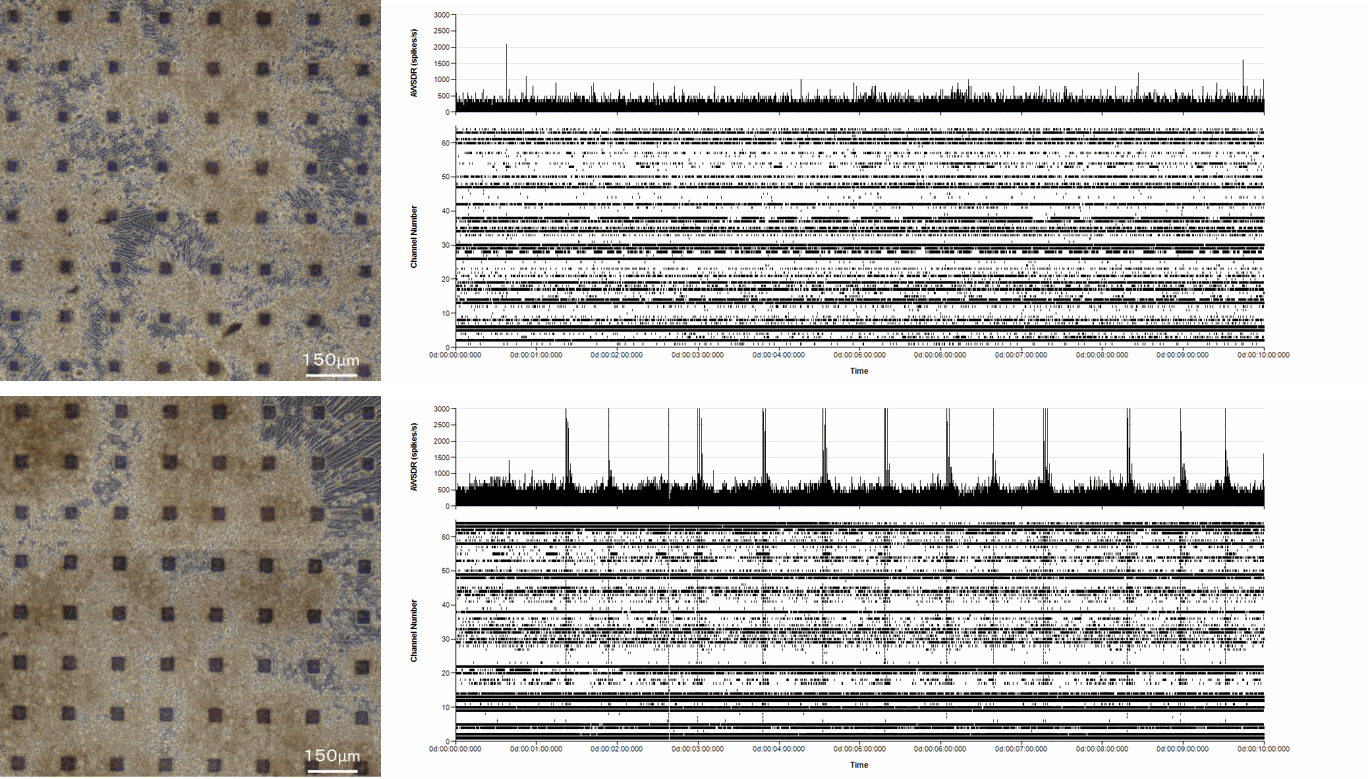
Axol Human iPSC-derived Sensory Neuron Progenitors show spontaneous extracellular field potential on a 64-channel MEA (Alpha MED Scientific) after 33 days in culture. Cells were seeded at 5x105 cells/cm2 . Data provided by Prof. Ikuro Suzuki (Tohoku Institute of Technology).
Drug treatment
Chemotherapy-induced neuropathic pain in the peripheral nervous system
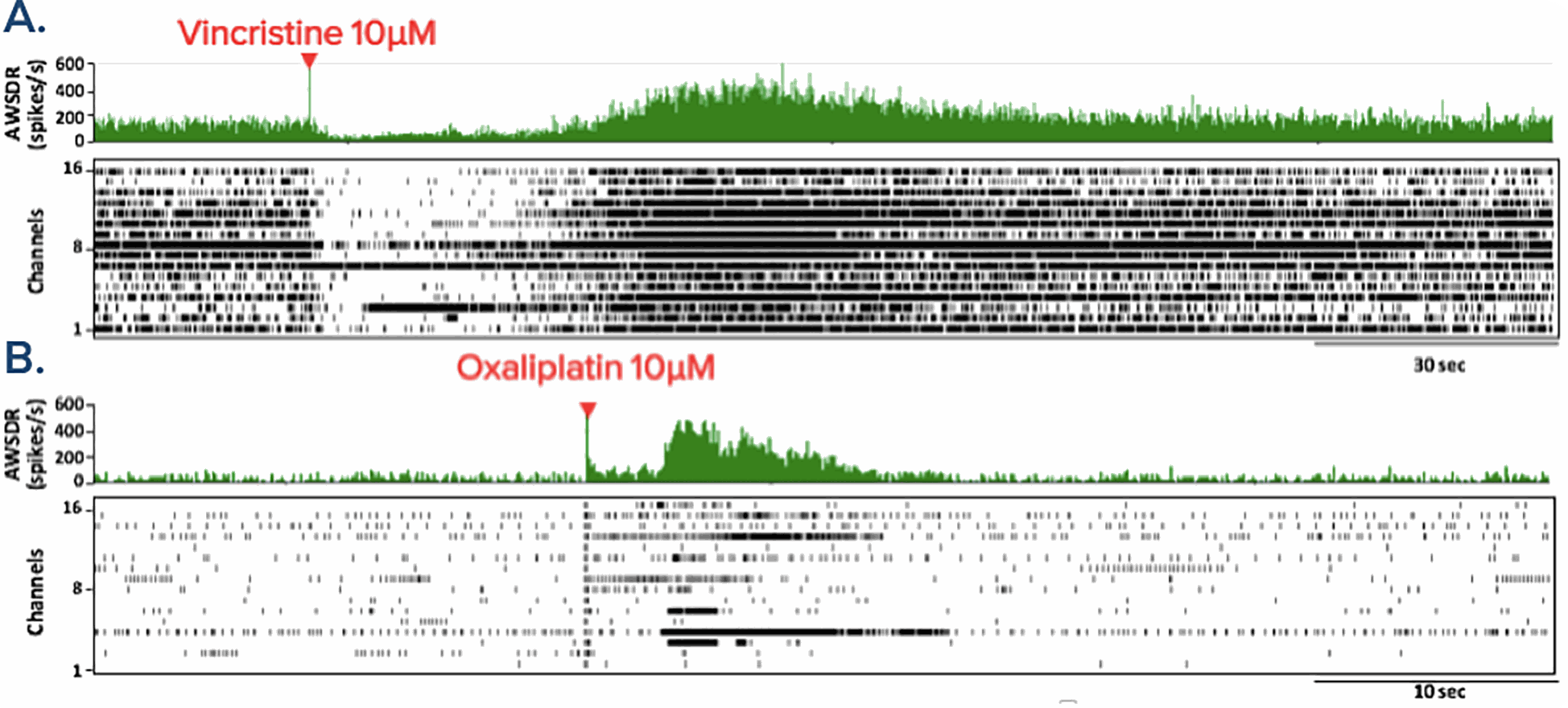
Functional Characterization of Neuropathic Pain: Chemotherapy drugs, vincristine and oxaliplatin, can result in the toxic side effect of peripheral neuropathy. The application of both of these chemotherapy drugs to hiPSC-sensory neurons resulted in an acute increase in firing rate, the time taken for the hiPSC-sensory neurons to respond was slow in comparison to the application of capsaicin, menthol and AITC. This image shows the rastor plot and array-wide spike detection rate (AWSDR) before and after administration of A. vincristine 10μM and B. oxaliplatin 10μM. Data provided by Prof Ikuro Suzuki (Tohoku Institute of Technology).
Oxaliplatin-induced cold hypersensitivity
Characterization of Hypersensitivity Induced by Chemotherapy Drug Oxaliplatin
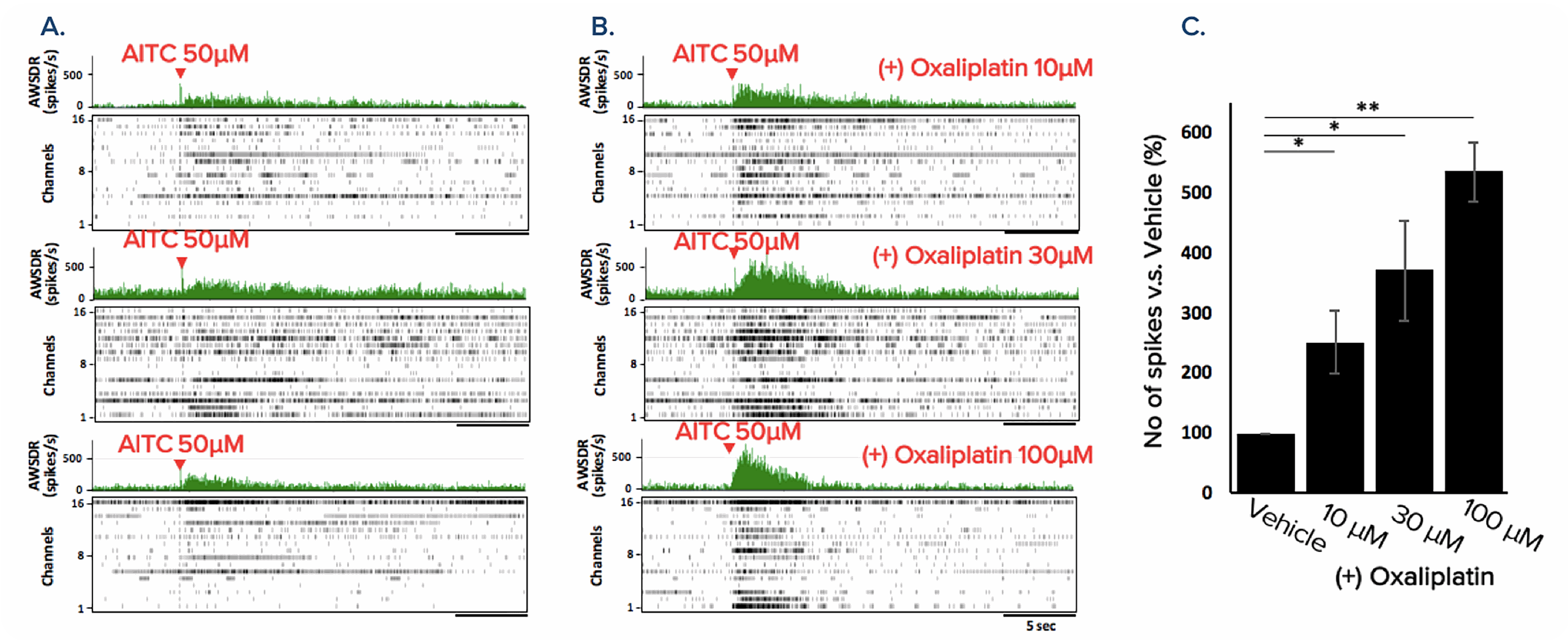
Characterization of Hypersensitivity Induced by Chemotherapy Drug Oxaliplatin: Oxaliplatin has further been implicated in the exacerbation of cold sensation. Here Oxaliplatin was shown to increase the firing rate of hiPSC-sensory neurons in response to AITC in a dose-dependent manner. Oxaliplatin results in a hypersensitivity to cold stimuli in hiPSC-sensory neurons. A. The response of hiPSC-sensory neuron to the application of AITC 50μM (control) B. hiPSC-sensory neuron treated with varying concentrations of oxaliplatin (10, 30 and 100μM), after 2 hours AITC 50μM was administered and the response was measured. C. Percentage increase of the number of firing spikes compared to the vehicle control. Treatment of oxaliplatin resulted in a dose-dependent increase in firing rate. (n=6, *p < 0.05, **p < 0.0005). Data provided by Prof Ikuro Suzuki (Tohoku Institute of Technology).
Resources
Protocol
- User Guide: Human iPSC-derived Sensory Neuron Progenitor
- - How to differentiate and mature Axol sensory neuron progenitors to function neurons.