Human iPSC-Derived Endothelial Colony Forming Cells

iPSC-derived endothelial colony forming cells
Endothelial colony forming cells (ECFCs) are rare, highly proliferative endothelial cells that are found in bone marrow and the bloodstream. ECFCs are highly angiogenic and can migrate to sites of blood vessel injury and contribute to repair of the damaged vessels. In partnership with Professor Mervin Yoder's laboratory at Indiana University, Axol has developed iPSC-Derived Endothelial Colony Forming Cells that resemble primary ECFCs in their cell surface marker expression and functionality.
Axol iPSC-Derived Endothelial Colony Forming Cells express the endothelial markers CD31, CD144, KDR and NRP1. The endothelial cells align in response to unidirectional shear flow and are responsive to pro-angiogenic factors secreted by fibroblasts. The ECFCs are stable and, unlike other iPSC-derived endothelial cell models, they do not transition to non-endothelial cell types during long-term culture. The endothelial colony forming cells can be passaged up to 10 times, permitting high throughput experiments that require large quantities of cells from the same donor. Axol iPSC-Derived ECFCs have comparable cell marker expression to primary HUVECs and have the advantage of greater consistency and the possibility of performing co-culture experiments with other cell types derived from the same iPSCs such as neural stem cells.
Why use iPSC-derived ECFCs
Reproducibility: Reduce the variability throughout your research with large batches sizes from a consistent source of endothelial cells from the same healthy donor.
Physiologically relevant: Expression of endothelial-specific markers (CD31, CD144, KDR, NRP1, eNOS, vWF) with typical cobblestone appearance.
Functionality: Comparable functionality to human primary cells as they form capillary structures in 2D and 3D culture and align to shear force. Our hiPSC-ECFCs have a range of proliferative potentials, perfect for screening compounds for anti- or pro- proliferative effects.
Highly expandable: Our hiPSC-ECFCs are stable up to 19 population doublings without loss of phenotype, making them suitable for high-throughput experiments.
Human iPSC-derived endothelial colony forming cells
Endothelial colony forming cells from healthy male and female donor iPSCs. The cells are cryopreserved at passage 4 and are provided at 1 million viable cells per vial. Axol iPSC-Derived Endothelial Colony Forming Cells can be stably expanded and repeatedly passaged in Axol Endothelial Colony Forming Cell Culture Medium.
| Cat. No. | Product Name | Starting Material | Quantity |
|---|---|---|---|
| ax2019 |
Human iPSC-Derived Endothelial Colony Forming Cells |
Human iPS cells |
1 million cells |
Endothelial colony forming cell culture media and reagents
| Cat. No. | Product Name | Quantity |
|---|---|---|
|
ax0044 |
Unlock |
25 mL |
| ax0049 |
Fibronectin Coating Solution | 1 m |
Characterization
Endothelial cell marker expression
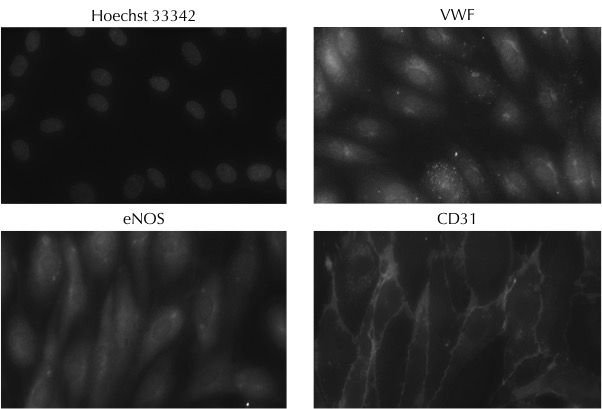
Expression of the endothelial cell-specific markers: von Willebrand factor, endothelial NOS and CD31 in Axol Human iPSC-Derived Endothelial Colony Forming Cells (ECFCs). Hoechst 33342 was used to visualize cell nuclei. The images were kindly provided by Dr Richard Siow (King's College London).

Expression of endothelial and lymph marker proteins in Axol iPSC-Derived Endothelial Colony Forming Cells as determined by flow cytometry. The cells demonstrate a clear endothelial cell phenotype and the expression of LYVE1 suggests the potential for differentiation to lymphatic endothelium. The dashed lines indicate negative control fluorescence levels and the solid lines represent the fluorescence of stained iPSC-Derived Endothelial Colony Forming Cells . The data were kindly provided by Dr Wolfgang Holnthoner (Ludwig-Boltzmann-Institute for Experimental and Clinical Traumatology).
Co-culture
Co-culture of iPSC-Derived Endothelial Colony Forming Cells with lung fibroblasts to assess angiogenic potential
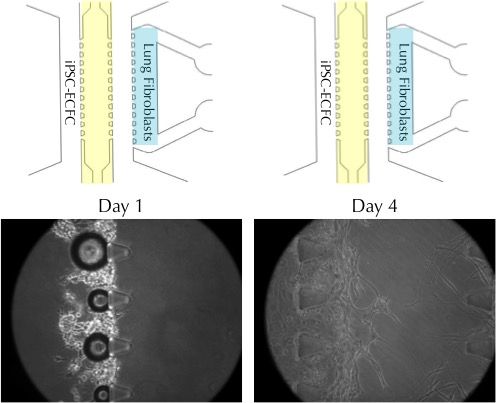
Axol iPSC-Derived Endothelial Colony Forming Cells (iPSC-ECFC) were seeded into a co-culture vessel together with human lung fibroblasts. By day 4, the endothelial cells grow towards the lung fibroblasts in response to secreted angiogenic factors. The process can be used to model the formation of perfusable blood vessels during angiogenesis. The images were kindly provided by Dr Jungho Ahn (Seoul National University).
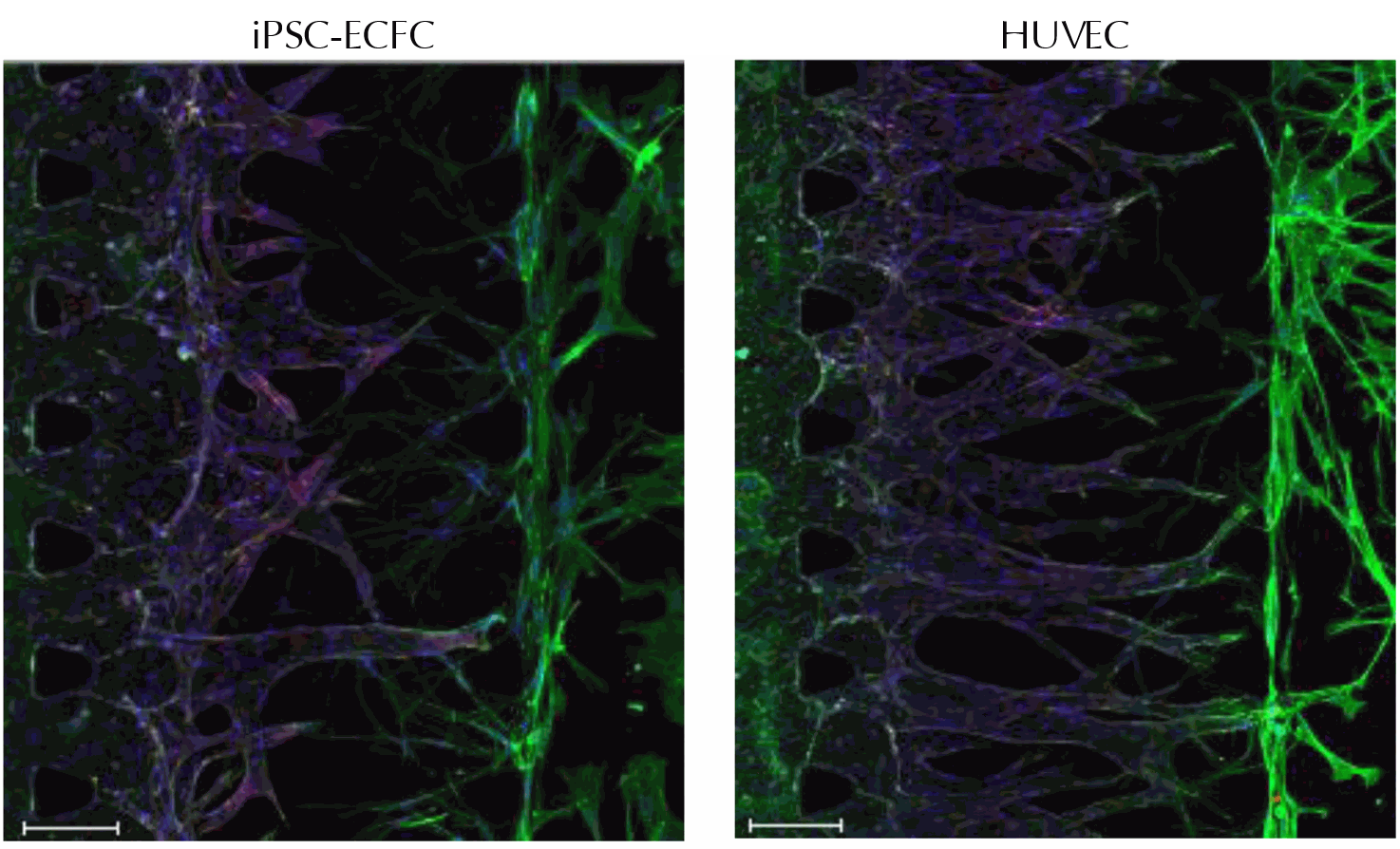
Axol iPSC-Derived Endothelial Colony Forming Cells extend outgrowths towards human lung fibroblasts when co-cultured. Primary human umbilical vein endothelial cells (HUVECs) were included as a positive control. The outgrowths were fixed and stained for CD31 (red), F-actin (green) and DAPI (blue). Scale bar = 150 μm. The images were kindly provided by Dr Jungho Ahn (Seoul National University).
Co-culture of iPSC-Derived Endothelial Colony Forming Cells with adipose tissue-derived stem cells to assess formation of vascular networks
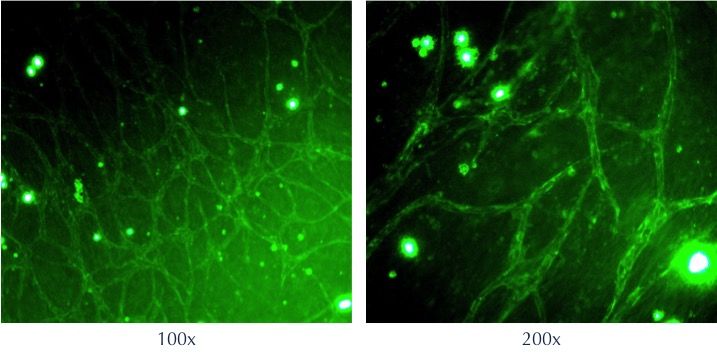
Axol iPSC-Derived Endothelial Colony Forming Cells were co-cultured with adipose tissue-derived stem cells in a fibrin clot (fibrinogen + thrombin). After 7 days, the cells have started to form a vascular network. The fibrin clots were fixed and stained for CD31 (green). The images were kindly provided by Dr Wolfgang Holnthoner (Ludwig-Boltzmann-Institute for Experimental and Clinical Traumatology).
Endothelial cell alignment
Response of iPSC-Derived Endothelial Colony Forming Cells to shear flow
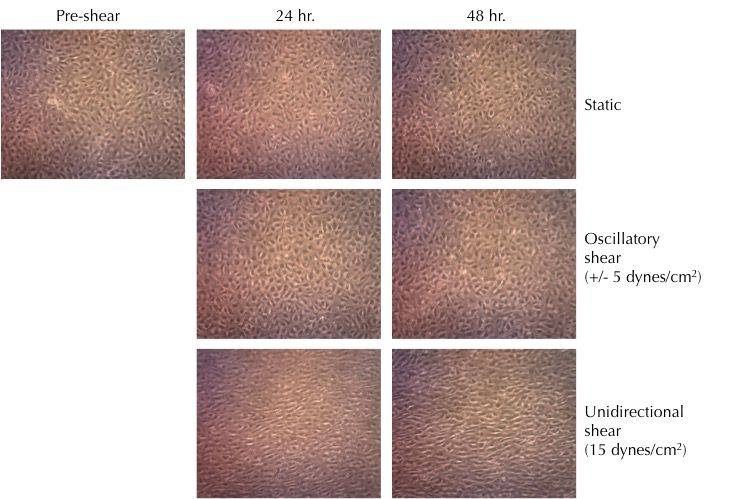
Axol iPSC-Derived Endothelial Colony Forming Cells were cultured in microfluidic slides under oscillatory or unidirectional shear flow. The endothelial cells responded to unidirectional shear flow by aligning. In static and oscillatory shear conditions, the endothelial cells did not align. The images were kindlymar provided by Dr Richard Siow (King's College London).
Angiogenesis
Axol iPSC-Derived Endothelial Colony Forming Cells seeded into Matrigel plugs form extensive 3D tubule networks after 24 hours in vitro.