iPSC-derived Striatal Neurons
Striatal neuron culture media and reagents
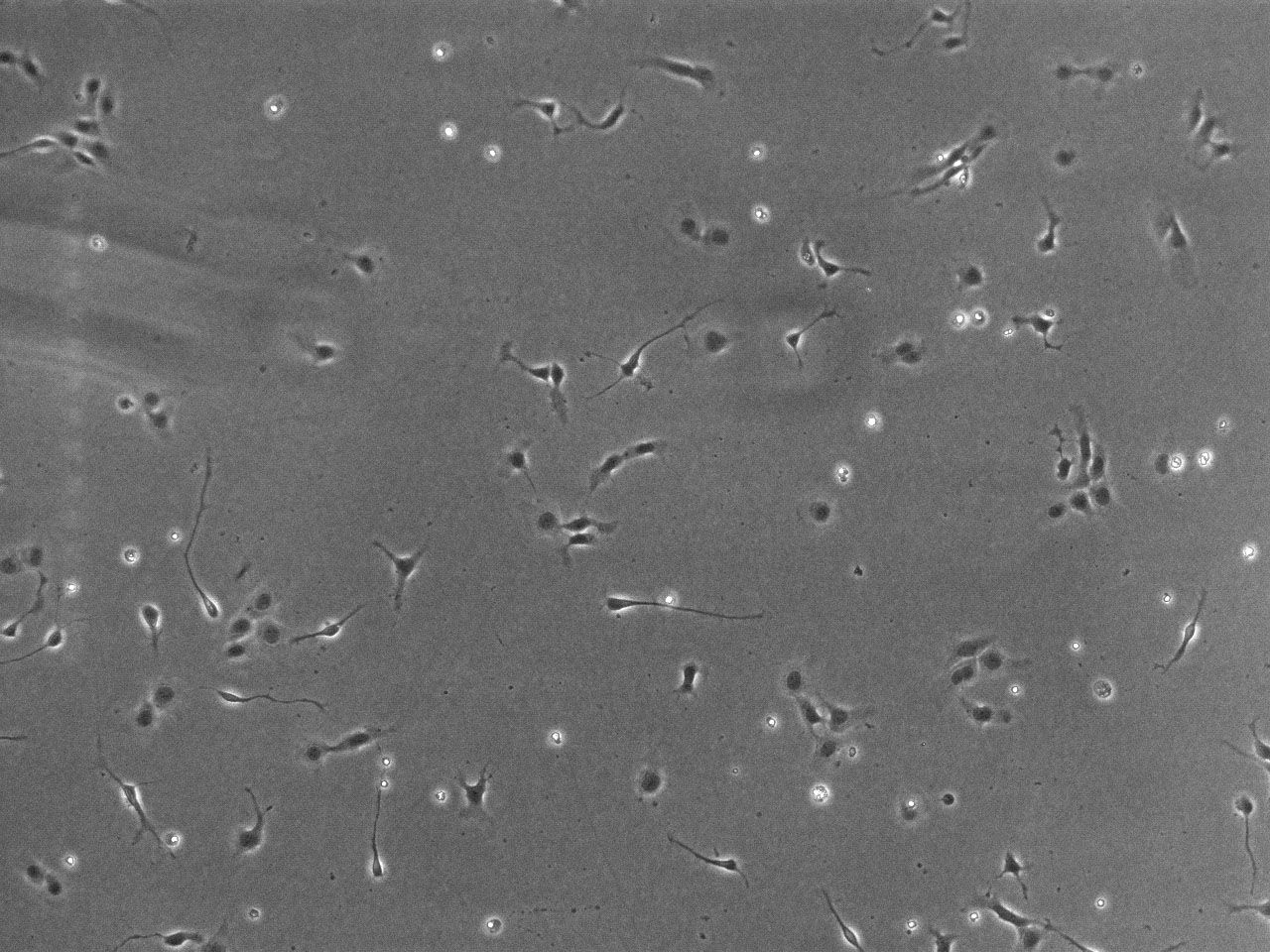
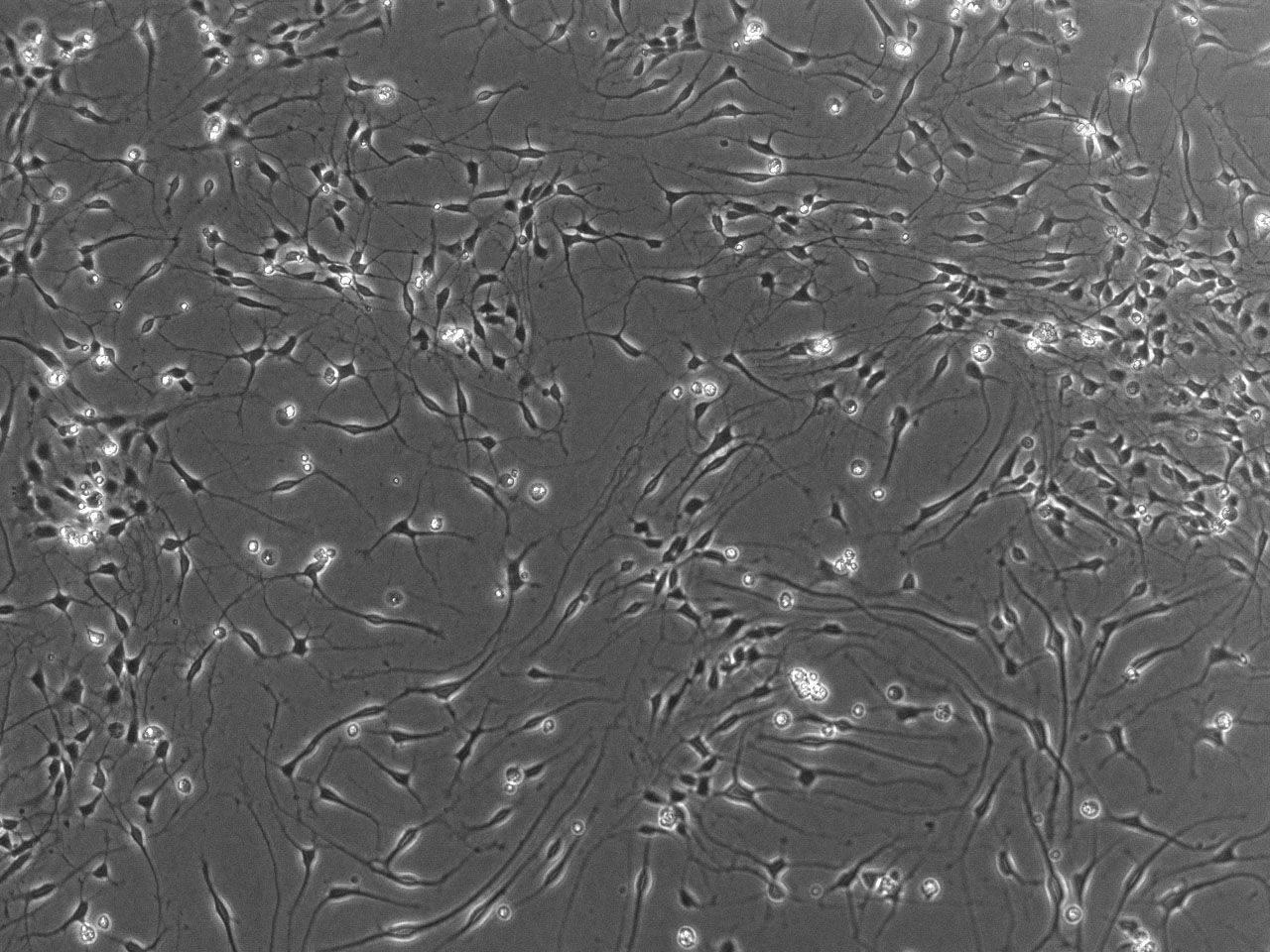
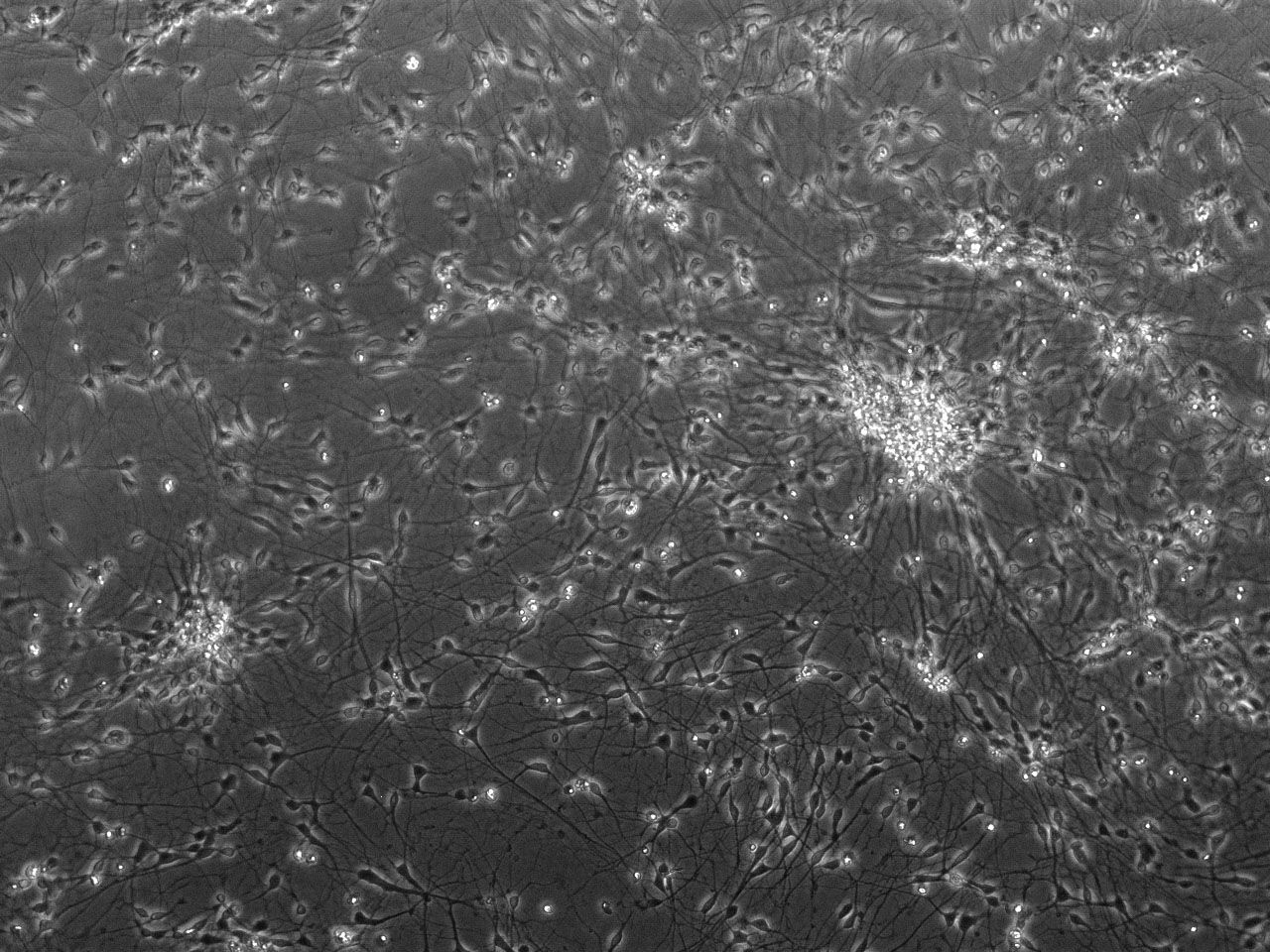
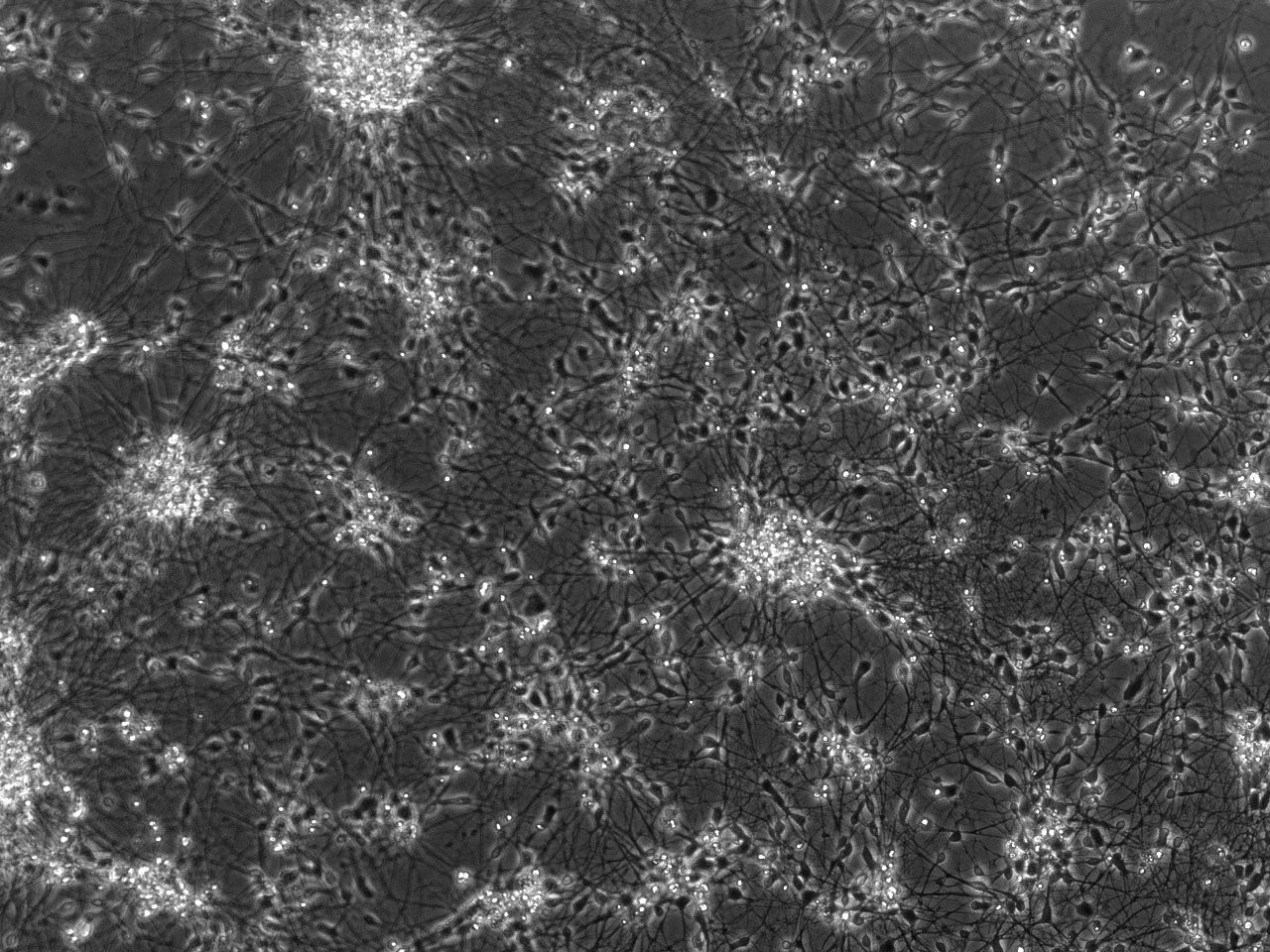
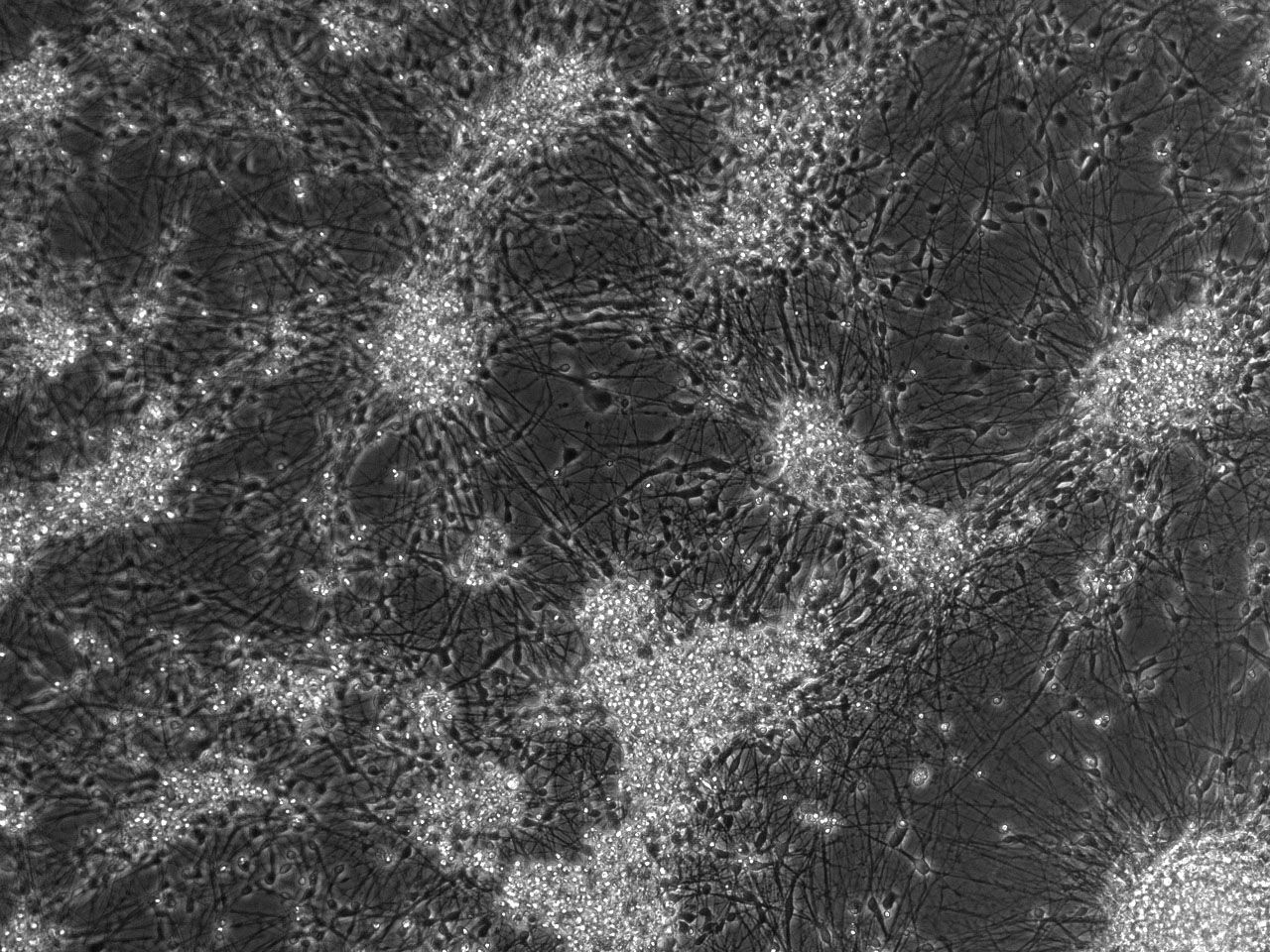
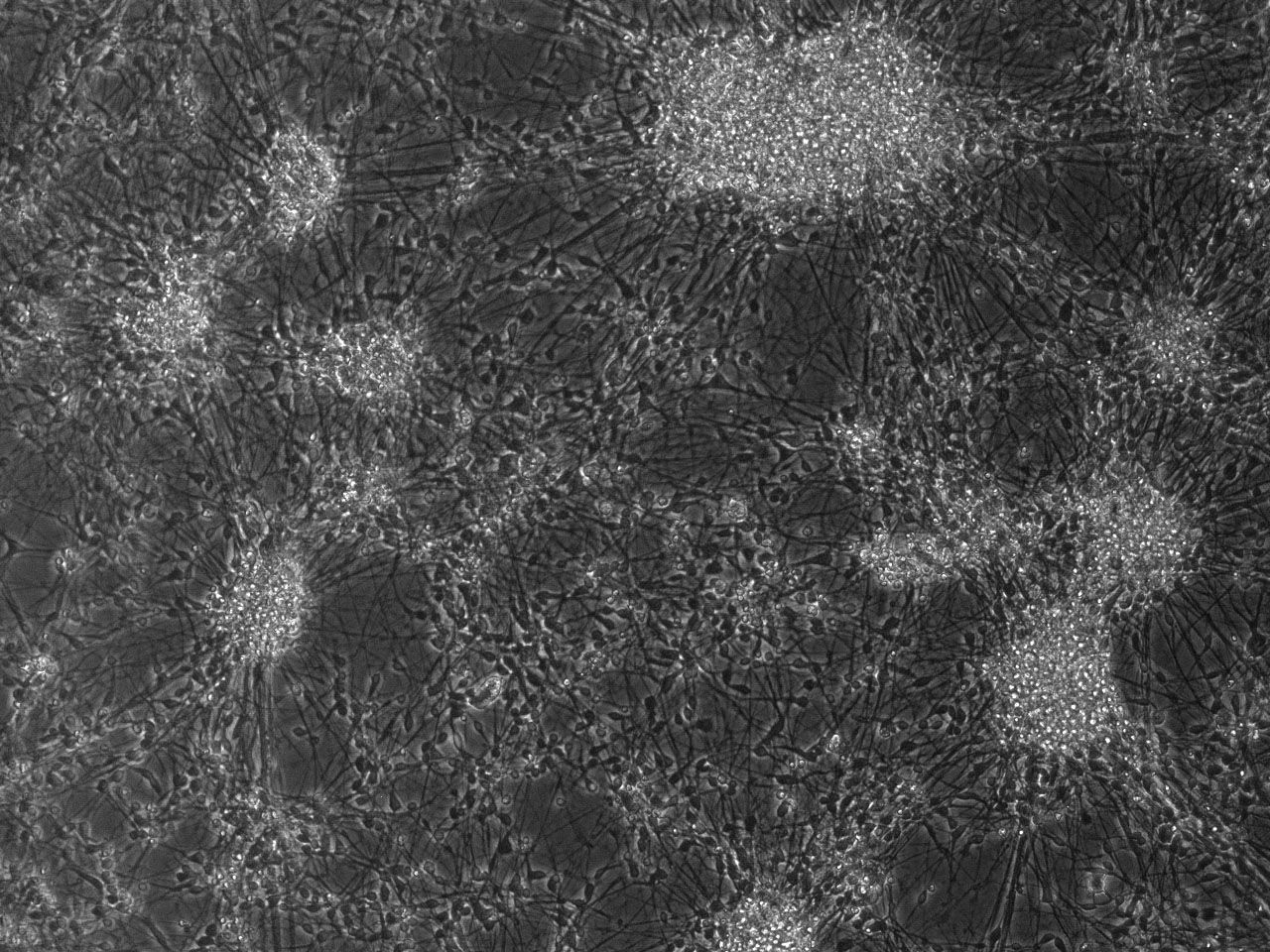
Phase contrast images of differentiating striatal neural culture
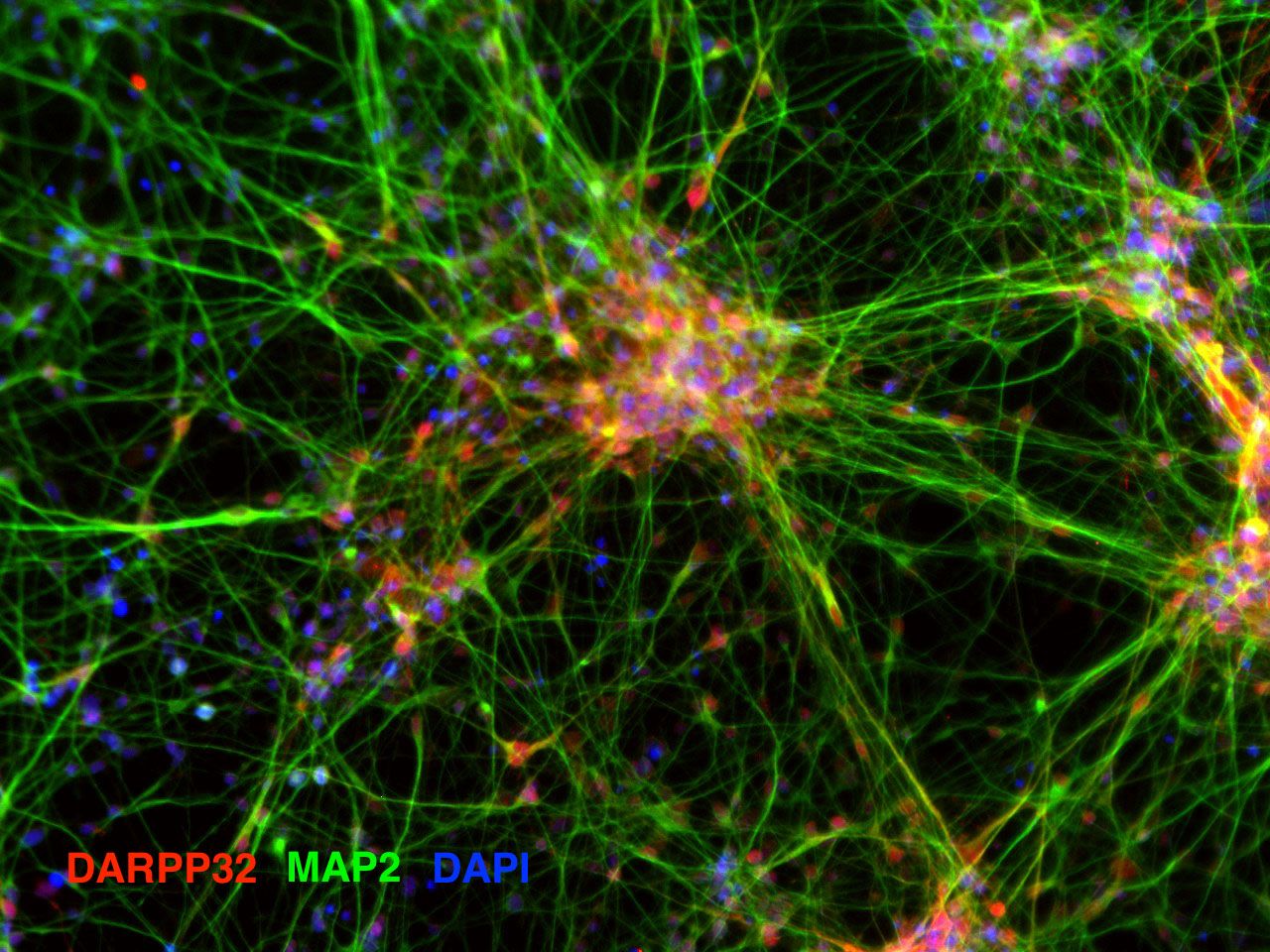
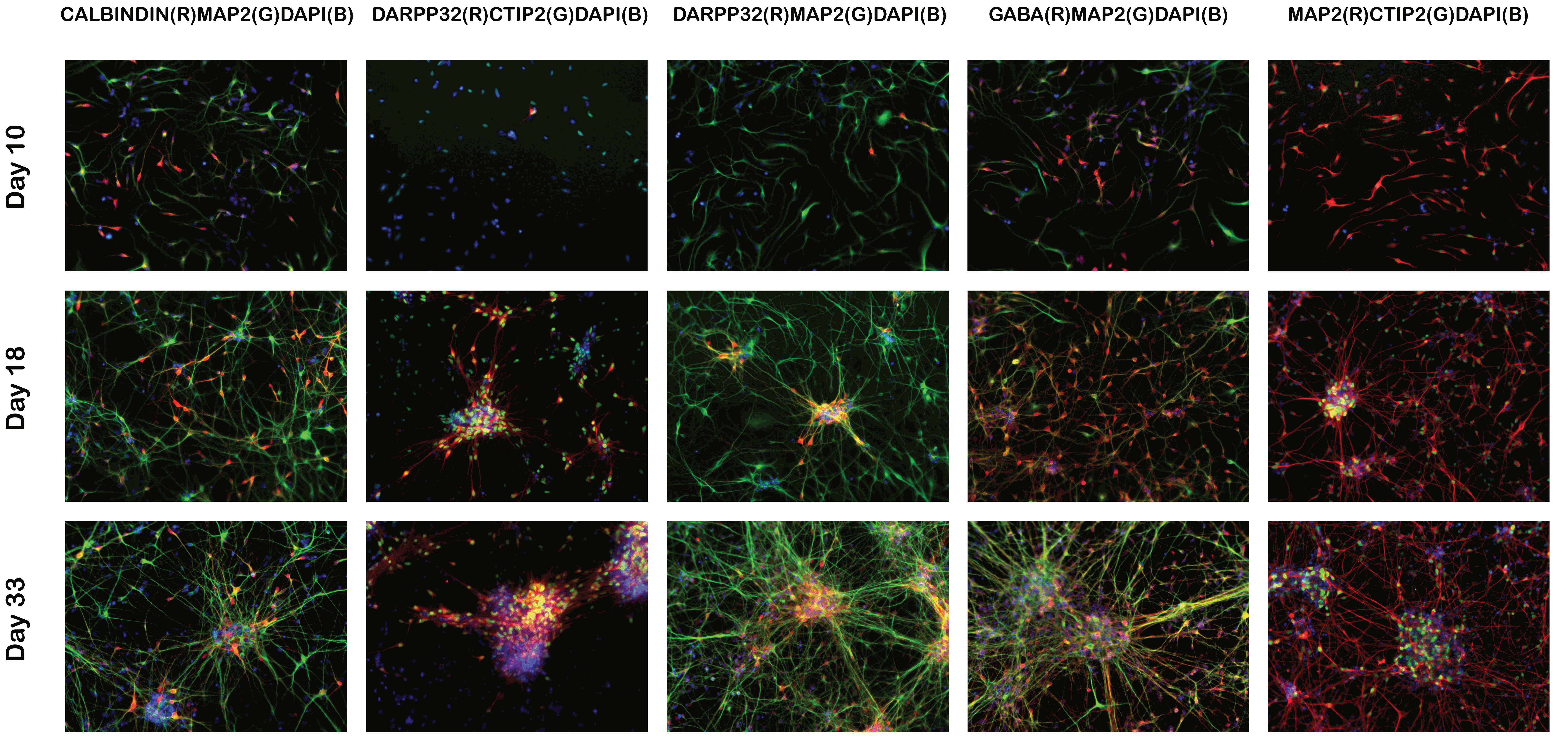
Validation of key marker expression by immunocytochemistry
ICC images of differentiating striatal neural culture. Cells were fixed and stained on day 10, day 18 and day 33 for markers of striatal neurons (medium spiny neurons): DARPP32, CTIP2, CALBINDIN, GABA and MAP2. Download PDF file showing additional ICC images.
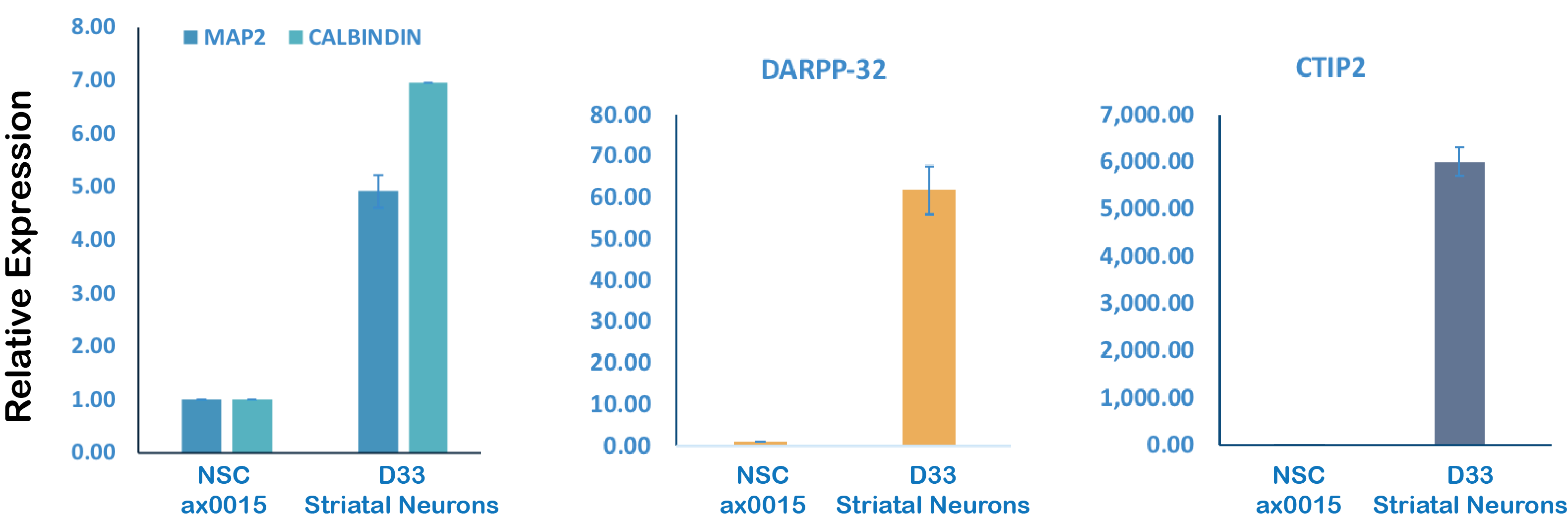
Quantitative real time PCR analyses of key marker expression levels
hCalbindin_F TGTGCGAGAAGAATAAACAGGT
hCalbindin_R TAAGAGCAAGATCCGTTCGG
hCTIP2_F CTCCGAGCTCAGGAAAGTGTC
hCTIP2_R ATGAGTGAGGGTGGGAGGAG
hDARPP32_F GAGATGGAGGCTCTGAGGAC
hDARPP32_R GGAAGGGCGCTGAGGTTCCT
hMAP2_F CGAAGCGCCAATGGATTCCC
hMAP2_R TGAACTATCCTTGCAGACACCT
Lactate Dehydrogenase (LDH) Cytotoxicity Assay
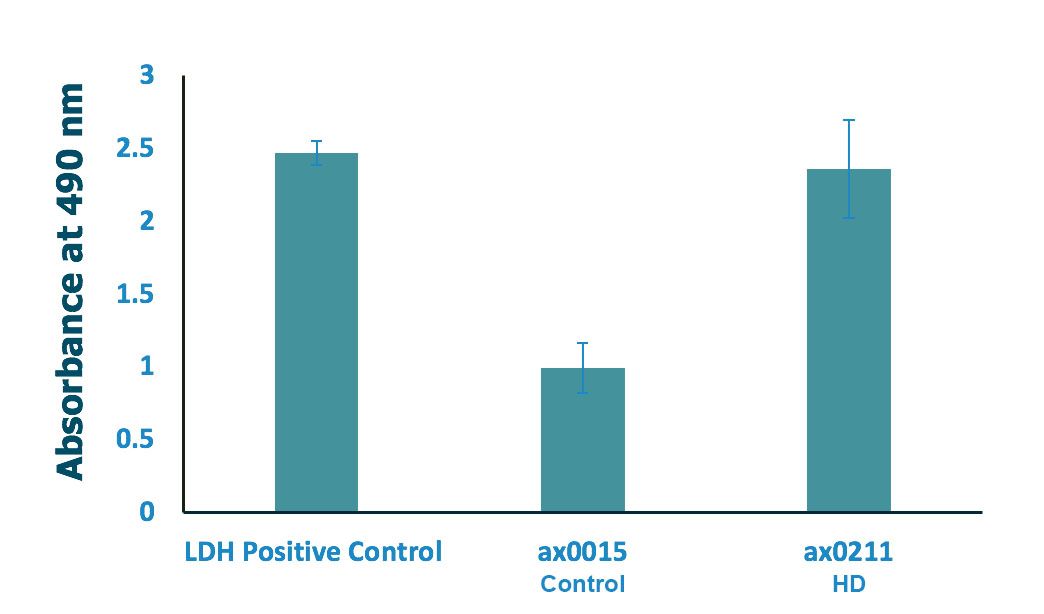
Significant increase in HD Striatal neuron cytotoxicity
Both iPSC-NSCs (ax0015 and ax0211) were seeded at 35,000 cells/cm2 and differentiated to striatal neurons in 96-well plate for 33 days. Cytotoxicity was measured based on LDH (lactate dehydrogenase) release. LDH activity was determined by spectrophotometric absorbance at 490 nm; n=3 (3 wells per condition), along with negative and spontaneous LDH release controls
Kit used: Pierce LDH Cytotoxicity Assay Kit. ThermoFisher Cat. No. 88953
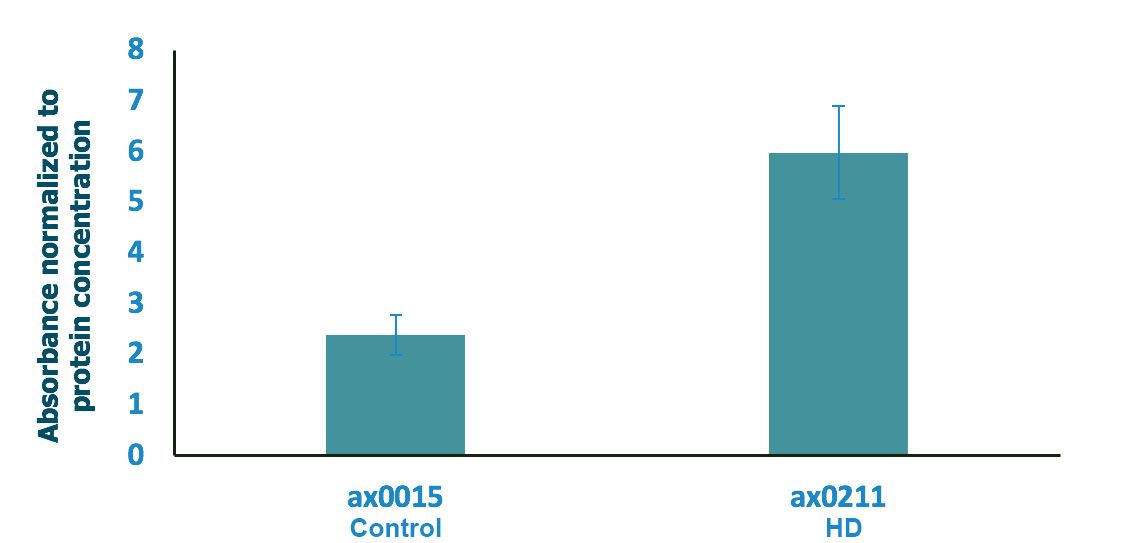
Absorbance normalized to the total protein concentration
The absorbance was normalised to the protein concentration post lysis (protein concentration was determined using Qubit 2). Normalisation did not change the results since the protein concentration of all samples were comparable.
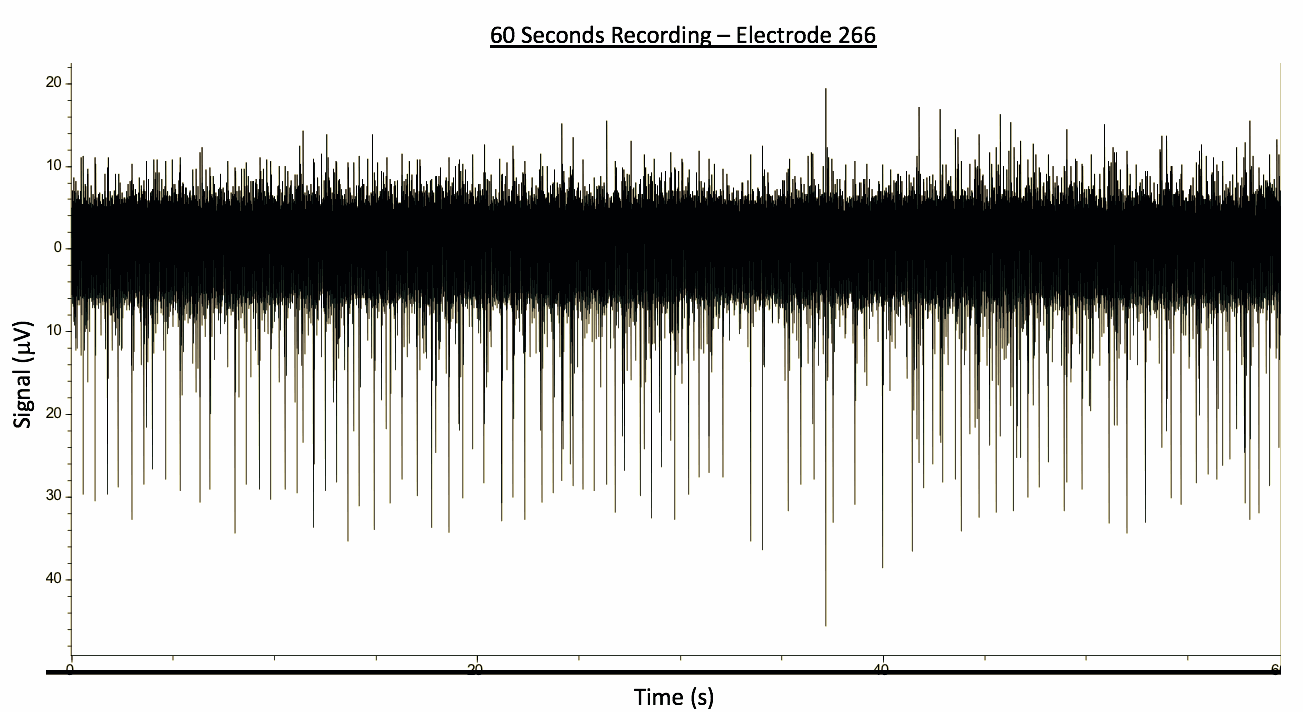
Electrophysiological characterisation using multi-electrode arrays
iPSC-derived NSCs (ax0016) were differentiated to striatal neurons using Axol Striatal Neuron Medium Kit on a 24-well multi-electrode array plate (AlphaMed Scientific Inc.) and spontaneous action potential recordings were carried out and analysed using MED64 Presto system.
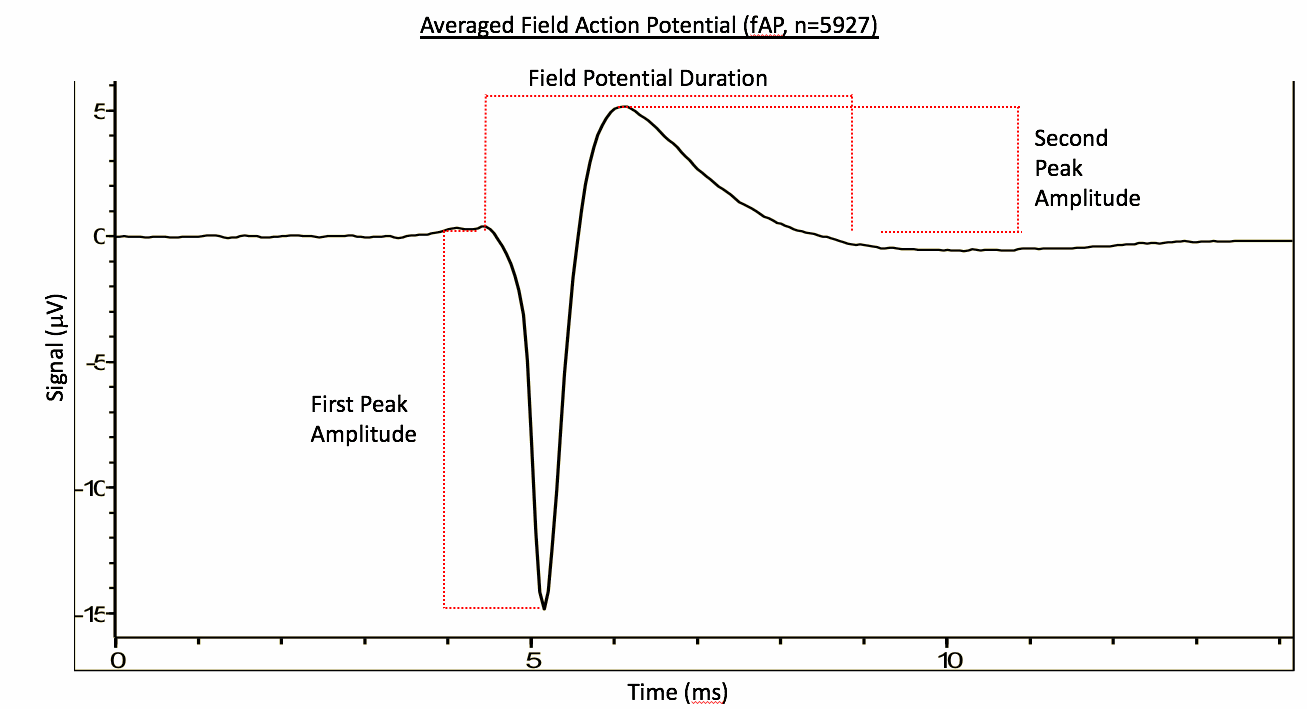
Spontaneous field action potential (n=5927)
Average Field Potential Duration: 3.66 ms
Average First Peak Amplitude: -16.69 uV
Average Second Peak Amplitude: 9.05 uV
60 second MEA recording
No. of bursts: 913; Burst rate: 1.52 Hz; Mean IntraBurst Interval: 34.96 ms
Frequency – Spike Rate within bursts: 420 Hz; Inter-Burst Interval (time between bursts): 527 ms
Burst First Peak Amplitude within bursts: -16.07 uV
Burst Second Peak Amplitude within bursts: 9.14 uV
Resources
- User Guide: Axol Striatal Neuron Medium Kit
- For generation of mature functional striatal medium spiny neurons
- To be used with Axol iPSC-derived NSCs: ax0015, ax0016, ax0018 and/or ax0211
-
Frequently Asked Questions
-
1. How long do I need to differentiate my NSCs before they are ready for striatal neuron-based assays?
-
The cells are ready for assaying on day 33. We recommend carrying out striatal neuron-based assay between day 33 and day 40.
-
-
2. How much medium and coating would I need for differentiating a vial (1.5 million cells) of NSCs to striatal neurons (Day 33) ready for assaying?
-
ReadySet: 12 mL
SureBond: 90 uL
Striatal Neuron Medium: 85 mL -
-
3. Can I make up fresh complete medium on a weekly basis?
-
Yes
-
4. Have you culture the cells on 96-well plates, if so, which supplier's plate do you recommend for use? -
We have differentiated and cultured our iPSC-striatal neurons on Greiner Bio-one's blackwalled 96-well plates (Greiner Item No. 655090)